trial interactive etmf|veeva vault etmf user guide : Baguio Our electronic trial solutions simplify your clinical operations: expedite timelines, . Incorrect code data. Check if you copied all the signs of the combination. Expired code. Each promo has an expiration date until it is valid. Don’t forget to see it before use. Wrong game or bet. Some codes were aimed at particular games and bet types. So, if you try to apply them to other entertainment, it might simply not work. .
trial interactive etmf,See the future of eTMF, today. Request a personal demonstration of the eTMF that study teams love. Learn about the industry's best eTMF clinical trial software. With over a .
Trial Interactive provides the TMF guidance and resources you need to stay .myTI is the mobile app for the Trial Interactive e-clinical platform, ensuring .
Our electronic trial solutions simplify your clinical operations: expedite timelines, .Our CTMS software enables remote oversight and powerful interoperability .eTMF. A practical, secure, compliant single access point for TMF documentation, .eTMF. A practical, secure, compliant single access point for TMF documentation, supporting all essential document processes and reducing the time, costs, and risks of TMF .
Trial Interactive provides the TMF guidance and resources you need to stay compliant. Join the thousands of clinical research professionals who use our TMF services to .Trial Interactive. © 2024 trial interactive. All Rights Reserved. Trial Interactive is a leader in clinical interactive document solutions and trial management solutions. Learn about the best eTMF in the industry, supported by expert TMF services.Trial Interactive’s industry-leading eTMF technology provides an award-winning user experience and ensures a constant state of compliance. Developed by clinical .Unfortunately your browser or operation system is not supported. We support Internet Explorer 10 or higher running on Windows that is newer than Windows XP.What is an eTMF? eTMF stands for electronic trial master file. It is the all-important trial master file in a digital format. Over the past decade, it has become standard in the pharmaceutical and biotechnology industries to .myTI is the mobile app for the Trial Interactive e-clinical platform, ensuring uninterrupted access to the eTMF for study teams. CRAs, site personnel, and sponsors can save valuable time by eliminating the need for .Our electronic trial solutions simplify your clinical operations: expedite timelines, enable collaboration, improve quality, capture the full story of study, and pass inspections. eTMF and TMF Services
Our CTMS software enables remote oversight and powerful interoperability with site feasibility, study start-up, eTMF, LMS, content management, and collaboration. Essential Features for a Modern Mobile-Friendly CTMS .Trial Interactive provides the most comprehensive clinical trial software that supports author-to-archive processes from site identification to study closeout. . “We looked at many eTMF systems, but felt Trial .
An eTMF platform provider with enough years in the industry will know best how to help you get up and running. Always try your meal before you start adding salt and pepper. . TransPerfect’s Trial Interactive is an .trial interactive etmf veeva vault etmf user guideThe 21 CFR part 11 compliant unified platform delivers an author-to-archive collaboration experience with solutions for clinical document management, site selection, site activation, e-learning, compliance training, quality, and more with seamless solution interoperability and indexing to the eTMF. Trial Interactive is consistently selected by .
veeva vault etmf user guidemyTI is the mobile app for the Trial Interactive e-clinical platform, ensuring uninterrupted access to the eTMF for study teams. CRAs, site personnel, and sponsors can save valuable time by eliminating the need for scanning, uploading, .
Trial Interactive TMF Expert Services scanned, coded, QCd, and merged all of the paper documents into the eTMF. All missing essential documents were recovered; Seamless onboarding of 4 CROs with controlled access to TMF; Trial Interactive increased document visibility, transparency, and control through KPIs and dashboards leading to a more .Scale your trial master file document processing. Stay Inspection Ready. With millions of documents processed to date, 22 TMFs rescued mid-study, and an ongoing tally of FDA, MHRA, and EMA inspections successfully completed, your TMF is in the most vetted and experienced hands, led by long-time industry professionals and TMF Reference Model .
Trial Interactive’s industry-leading eTMF technology provides an award-winning user experience and ensures a constant state of compliance. Developed by clinical professionals for clinical professionals, our eTMF enables inspection readiness, increases visibility and oversight for key stakeholders, and improves collaboration for more productive study .

By using the website, you accept this policy. GlobalLearn is Trial Interactive's industry learning learning management system connected to content management and eTMF solutions. Create elearning programs from study documents. Automatically index learning certificates in the eTMF.Automate essential TMF processes, simplify oversight on every step in the clinical trial journey, and maintain constant inspection readiness. Manage amendments, milestones, and any other eTMF changes with TI’s new Events Manager. Export audit trail reporting that provides information on all documents in a room with instant audit trails.TransPerfect’s Trial Interactive is an industry leader in practical, global eClinical innovation that simplifies and automates clinical processes for sponsors, CROs, and sites around the world. By using the website, you accept this policy. Implementing an eTMF for the first time can feel like a daunting process. This checklist will help you .The interoperability between Trial Interactive’s eISF, eTMF, and LMS solutions enables full visibility into all documents needed to remotely oversee a trial, centralizing essential documents and certified copies of source documents while securing protected health information. Sites can even upload and generate certified electronic copies of .
This journey began with the Trial Interactive eTMF and emerged as an early influencer and leader in digitizing clinical operations. Now, the Trial Interactive platform has evolved to enable Pharma and Biotech Sponsors, and CROs to improve speed and quality in one seamless experience. Study teams can streamline processes across site .eTMF to Trial Interactive’s secure file share. Trial Interactive monitors the secure file share as you upload your data. 3. TI Creates the Target Room No action needed, but be prepared to answer questions we may have about your data. Trial Interactive builds a standard eTMF room based on the 3.2.0 TMF Reference Model (CDISC). 4.
Trial Interactive is TransPerfect’s eClinical platform to simplify clinical content management and oversight. From site identification to close-out, clinical document processes and operational oversight are complex. Our electronic trial solutions simplify your clinical operations by expediting timelines, enabling collaboration, improving .
Workshops are a fast and efficient method for exchanging knowledge and engaging in interactive problem-solving. With millions of documents processed to date, 40+ TMFs rescued mid-study, and an ongoing tally of FDA, EMA, MHRA, PDMA, and GCP inspections successfully completed, long-time industry professionals and TMF Reference Model .

Trial Interactive Tapped as eTMF Provider PHILADELPHIA, September 5, 2012 – Premier Research announced today that it will use a new electronic Trial Master File (eTMF) system on all Clinical Development Services Studies. Trial Interactive has been tapped to provide the supporting platform. The adoption of eTMF is the .Here’s an overview of how we make migration easy for our clients in six steps. With our experts at your side, you can ensure that your organization’s migration process will be seamless, so you can focus on what matters most—your research. Download the Guide. The 6-Part Checklist to Switch to an eTMF Your Team Will Love. Prepare for Your .
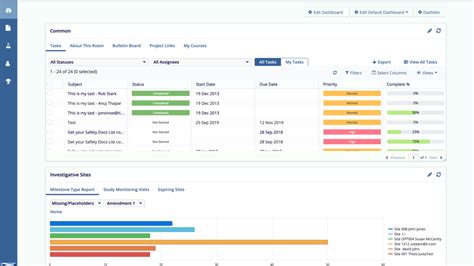
trial interactive etmfThe informative dashboards, KPIs, and reports. provided by our eTMF interface allowed for a clearer picture of the overall health of the TMF moving forward. Transperfect’s Trial Interactive eTMF could be configured to the specific workflows required of the company to increase oversight and control.
trial interactive etmf|veeva vault etmf user guide
PH0 · veeva vault tmf viewer
PH1 · veeva vault etmf user guide
PH2 · trial interactive transperfect
PH3 · trial interactive tmf
PH4 · transperfect trial interactive etmf
PH5 · transperfect etmf
PH6 · etmf clinical trial
PH7 · electronic trial master file software
PH8 · Iba pa